QUT - Capstone Project (March - Present 2024)
Product Design & Testing: Oxygen Concentrator for developing nations
Scroll ↓
Purpose of an Oxygen Concentrator Device
Provides air at higher than atmospheric oxygen purities, helping patients breathe, reducing strain on respiratory muscles, and helping them maintain adequate blood oxygen levels
Project Aim
Design, construct, and optimize a portable oxygen concentrator (POC) device following the need:
A way to deliver a consistent supply of concentrated oxygen to improve the quality of life for children in the South Pacific with pneumonia and without access to a hospital.
General Overview: Research & Proposal —> Design —> Experimentation and CFD Simulation —> Optimize dependent variables —> Refine Design (MVP) —> Pilot Prototype
Project Overview
Documentation
Market analysis (Determine and establish end-user requirements)
Review stakeholders and resources
Assessment of risks, requirements & constraints
Framework for assessing quality and sustainability
Outline of deliverables, linked to a project timeline
Proof-of-concept design
Specifications
Need specifications were established, defining appropriate parameters and constraints related to the design
Group of three, supervised by Prof. Cameron Brown (Director of Medical Engineering Research Facility QUT)
Project split into three key areas, with my role focusing on:
Development and Implementation of a method that synchronizes key components to concentrate oxygen at a low cost, focusing on power availability, parts accessibility, and environmental conditions
Context / Methodology
Humanitarian approach:
Clinical stakeholder Dr. Kiran Shikar advised separation from Western-centered International Standards, and instead, focus on a novel “Bulk Air” approach where the need for flow rate supersedes that of oxygen purity
“Bulk Air”, sacrificing oxygen purity, and increasing flow rate, aiming for 55-66% and >4LPM respectively
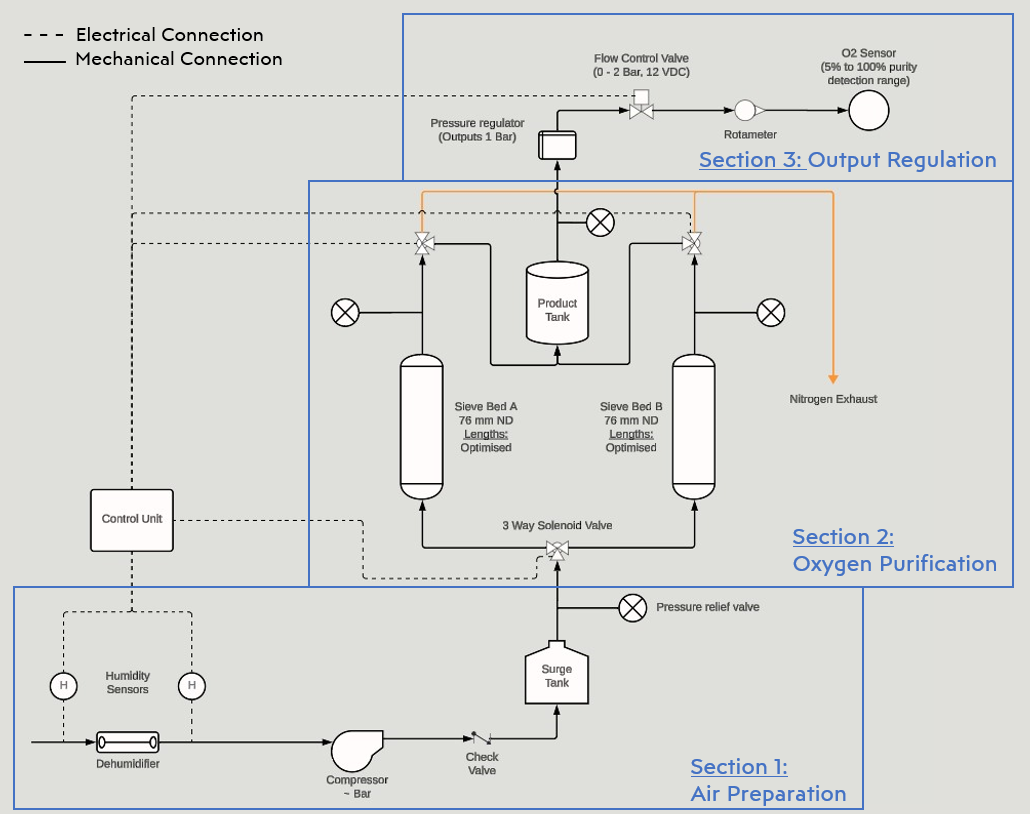
General project process outlined in Figure 1. Key milestones related to my responsibility:
Electrical & Mechanical design based on justified assumptions and design criteria
Initial Test: Feasibility of design approach and Optimization of sieve bed sizing and pressure
Adaptation with modeling results and defined performance requirements
Second Test: Ensure full-system functionality and Iterative refinement of design
General project methodology [Fig.1]
Inputs and Controls
Input ranges for testing models
Control Components
Control Unit Assembly
Testing Models
Both models will conduct experiments tailored to producing a minimum viable product (MVP), through a novel air purification approach: Propagating Pulse Adsorption (PPA).
Multi-Bed Model (Second Test)
Provides a refined understanding of an oxygen concentrator device
Purpose of testing full-system functionality after parameter optimization
Note: End product will include safety/design requirements aligning with medical regulations
Simplified for ‘Initial Test’
—>
Single-Bed Model (Initial Test)
Focuses on feasibility then optimisation of PPA parameters
Tests viability of PPA novel approach —> Reduce to one sieve bed
Robust Labratory equipment —> Remove unnecessary end user and device safety components
— Project is still in progress (Expect the next update in August 2024) —
Testing and Design Adjustment
…
Examination methods: Theoretical (Datasheets), Simulation (COMSOL), Experimental (Laboratory)
Simulation
Theoretical
Experimental
…
Design Criteria:
Control Unit: Open-source, robust, and user-friendly
Power Supply: Stable, versatile and efficient
Compressor: Oil-free and low-cost considering maintenance requirements and vibration levels
Validation & Fabrication
…
Reflection
…
~…~